- Your cart is empty
- Continue Shopping
Ammonia Poisoning In Aquarium Fish – 2022

What Is Ammonia Poisoning?
Ammonia poisoning is one of the most common water quality problems diagnosed in aquaculture. Ammonia is the primary nitrogenous waste product of fish and also originates from the decay of
complex nitrogenous compounds (e.g., protein). Ammonia can cause acute mortality, but most often it presents as sub-lethal stress.
Aquarium
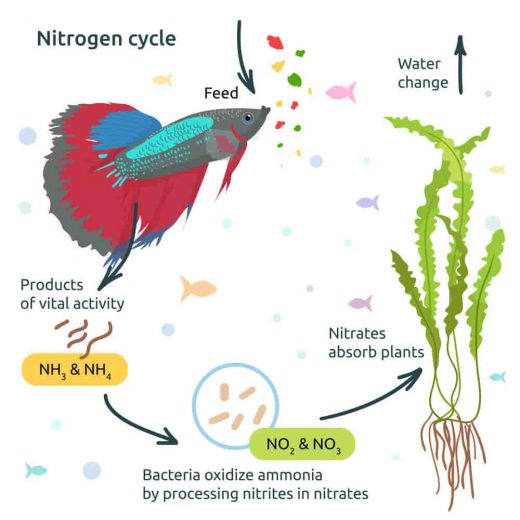
In an aquarium, ammonia accumulation is due to an inadequate number of bacteria that oxidize ammonia into nitrite. Nitrosomonas europaea is the predominant ammonia oxidizer in both freshwater and marine environments, but other Nitrosomonas species dominate under certain conditions. Nitrosococcus and
Nitrosospira species are also well-known ammonia oxidizers. These ammonia-oxidizing bacteria (AOB) are scarce in a new aquarium setup. So, when fish are added to the new tank, the ammonia rapidly rises, killing the fish; this is often referred to as new tank syndrome.
Ammonia poisoning can also occur in long-established aquariums. If fish are added to a tank that has much fish already present or if fish are overfed, causing an accumulation of decaying food in the tank, ammonia can rise. The total amount of ammonia that can be converted to nitrite depends entirely on the amount of biological filtration in the tank.
Biological filtration (more appropriately termed microbiological filtration, since it refers to
the filtration of water over microbes) occurs when the aquarium water passes over a surface coated with AOB. Thus, biological filtration (and ammonia removal) is greatest where there is a high water flow over a large surface area. This occurs in the aquarium’s filters (under gravel, box, and power filters). If the biological filtration capacity is too low to remove all the ammonia produced by the fish, ammonia will rise. If filters are cleaned too vigorously (e.g., gravel stirred excessively), it will cause an ammonia spike, since the bacteria are easily dislodged from the substrate and are susceptible to changes in environmental conditions.
Ponds
Ammonia is usually not a problem unless supplemental aeration is used, preventing environmental hypoxia and thus allowing higher fish densities. As in other systems, feeding (uneaten, decaying food and ammonia generated from food consumption or dead fish) is the largest source of ammonia in a commercial pond. Ammonia toxicity is most likely to occur near sunset, when pH, temperature, and thus unionized ammonia are at their peak.
In most ponds, algae, as well as Nitrosomonas bacteria, are major consumers of ammonia. Most ponds, especially commercial aquaculture ponds, have large algae populations. Ammonia also tends to increase during fall and winter, possibly because of a decrease in algal and bacterial metabolism at low temperatures. Ammonia may also rise after an algae crash or massive die-off; this not only reduces ammonia assimilation but also adds to ammonia buildup caused by the decaying algae. Algae die-offs can occur spontaneously or may be caused by algaecidal chemicals.
Diagnosis of Ammonia Poisoning
Ammonia levels are easily determined using commercially available kits. These kits measure the nitrogen
present as ammonia, also known as total ammonia nitrogen (TAN). An ion-specific electrode can also be used to measure ammonia. Ammonia is present in two forms: unionized (NH3 ) and ionized (NH4+). Unionized ammonia (UIA) is toxic to fish, while ammonium (NH4+) is much less toxic. The amount of UIA in water depends mainly upon the pH, and also on temperature and salinity. High pH and temperature and low salinity favor the presence of UIA.
Treatment of Ammonia Poisoning
AQUARIUM
- 25 – 50% water change (daily to weekly, depending on
ammonia concentration) - Add zeolite
- Add buffer to reduce pH (freshwater only)
- Add nitrifying bacteria
- Add biological filtration
- Decrease density
- Temporarily reduce or stop feeding
Monitor closely for possible nitrite increase
PONDS, FLOW-THROUGH SYSTEMS
- Stop or reduce feeding
- Decrease density
- Add water or increase water flow